Radiation Therapy for Cervical Carcinoma
Authors
INTRODUCTION
Radiation therapy plays a vital role in the management of carcinoma of the cervix. Carefully planned and executed radiation therapy yields excellent pelvic control and survival rates (Table 1).1, 2, 3, 4 Many stage IB1 cancers are treated equally effectively with radiation or radical surgery; most more advanced lesions are best treated with radiation. Although radiation alone can cure many patients with local-regionally advanced disease, recent studies have demonstrated that concurrent delivery of cisplatin-containing chemotherapy can improve the rates of local disease control and survival.5, 6, 7, 8, 9 However, treatment modality and technique must be individualized on the basis of clinical, anatomic, and social factors. Optimal treatment selection always requires close cooperation between the patient’s referring physician, gynecologic oncologist, and radiation oncologist.
Table 1. Results of treatment with radiation therapy for carcinoma of the cervix
| Study | FIGO Stage | No. of Pts. | Pelvic Control Rate (%) | 5-Year Survival Rate (%) | % Tumors≥ 6 cm | |
| Coia et al. (1990)2 | IB | 168 | 88 | 74 | ||
| II | 243 | 73 | 56 | |||
| III | 114 | 49 | 33 | |||
| Barillot et al. (1997)1 | I | 218 | 90* | 89 | ||
| IIA | 315 | 86 | 82 | 14.5 | ||
| IIB | 314 | 78 | 70 | |||
| IIIB | 482 | 57 | 49 | |||
| Perez et al. (1992)4 | IB | 384 | 94 | 90 | 13.5* | |
| IIA | 128 | 88 | 81 | 39.1* | ||
| IIB | 353 | 83 | 77 | |||
| III | 293 | 64 | 59 | |||
| MDACC (1980–1994)† | IB1 | 524 | 98 | 86 |
| 22.8 |
| IB2 | 482 | 81 | 67 | |||
| IIA | 149 | 84 | 67 | 20.5 | ||
| IIB | 211 | 81 | 54 | 58.8 | ||
| III | 328 | 70 | 47 | |||
| Lowrey et al. (1992)3 | IB | 130 | 81 | 22 | ||
| IIA | 164 | 74 | 5 | |||
| IIB | 112 | 64 | 43 | |||
CLINICAL PRESENTATION
Cervical carcinoma may present with a variety of anatomic configurations. As tumors enlarge, they can produce lesions that are primarily exophytic, infiltrative, or ulcerative. The anatomic distortion produced by these lesions is an important consideration in determining the optimal sequence of therapy. Large exophytic lesions usually necessitate an initial course of external-beam radiation therapy (EBRT) to shrink the tumor and allow close approximation of the intracavitary system to the cervix during subsequent intracavitary radiation therapy (ICRT). Lesions that originate in the endocervix may expand the cervix concentrically and become barrel-shaped. In such cases, initial EBRT is used to shrink the tumor so that it is within the range of the high-dose portion of the ICRT dose distribution. The tumor may spread beyond the cervix by direct extension, lymphatic involvement, or hematogenous metastasis. The tumor may extend directly to involve the vagina, uterine body, paracervical tissues, and, infrequently, the bladder or rectum. Microscopic vaginal involvement may extend some distance beyond visible or palpable disease, and palpable discontinuous vaginal involvement can occur when the disease spreads along submucosal lymphatic channels. Although the tumor may extend directly to adjacent tissues, discontinuous involvement may also occur by lymphatic metastasis to the lymphatics of the broad ligament. Tumor invasion of the periureteral tissues sometimes causes ureteral obstruction and hydronephrosis. Although the rate of regional metastasis is strongly correlated with tumor size and stage, even small cancers may involve regional lymphatics. The obturator, hypogastric, and external iliac nodes are most frequently involved. The tumor may then progress secondarily to involve the common iliac, precaval, and para-aortic nodes. Lymphatic spread to the presacral nodes also occurs via lymphatic channels in the uterosacral ligaments.
CLINICAL EVALUATION AND STAGING
The patient’s general medical condition and the extent of the tumor must be evaluated carefully before treatment is selected.
History and Physical Examination
Before treatment, patients should undergo a careful physical examination and a detailed history should be taken, with special attention to conditions such as diabetes, severe hypertension, and pulmonary and cardiac disorders. A history of multiple abdominal operations or pelvic inflammatory disease is associated with an increased risk of late treatment-related complications. Recent studies indicate that cigarette smoking is also correlated with an increased risk of late complications.10 Patients should be questioned about the amount and duration of any vaginal bleeding, and severe anemia should be corrected prior to treatment.
Review of Tumor Histology
Tumor histology should be reviewed carefully before a final treatment plan is selected. Most cervical tumors are squamous. Although these tumors have been subdivided in terms of cell size, grade, and the degree of keritinization, the subtypes of squamous carcinoma seem to have little impact on prognosis. Approximately 20% of cervical cancers are composed partly or entirely of other histologic types, such as adenocarcinoma, adenosquamous carcinoma, or small cell neuroendocrine carcinoma.
In the past, authors argued that patients treated for adenocarcinomas and squamous carcinomas had similar survival.11 However, small numbers of patients and differences in the definitions of microinvasive disease for patients with adenocarcinomas and squamous carcinomas have complicated comparisons.12 Other authors have reported a poorer prognosis in patients treated for adenocarcinomas. In one of the largest studies comparing outcomes in women treated with radiation for adenocarcinomas or squamous carcinomas of the cervix, Eifel and coworkers12 reported histology to be an independent predictor of poor prognosis, with more frequent distant metastasis and poorer survival in patients with adenocarcinoma; pelvic disease control rates were similar for squamous and adenocarcinomas. The association between grade and prognosis is well established for adenocarcinomas.12, 13, 14 In contrast, there is no evidence of an association between grade and prognosis for squamous cell carcinomas.
Small-cell neuroendocrine cancers of the cervix are rare, comprising fewer than 5% of all cases. They are histologically similar to small cell neuro-endocrine carcinomas originating in other sites and frequently behave very aggressively, with rapid widespread dissemination.15, 16, 17 Lymph-vascular space invasion and lymph node involvement are frequently present at diagnosis. Although patients with small-cell carcinomas sometimes have dramatic responses to neoadjuvant chemotherapy,15 the impact of chemotherapy on survival has not been established, and there is no evidence that radiation therapy doses can be decreased without increasing the risk of local failure.
Determination of Extent of Disease
The staging system for cervical cancer recommended by the International Federation of Gynecology and Obstetrics (FIGO) is shown in Table 2. The system categorizes patients in terms of the extent of clinically detectable local disease. However, it does not include some important predictors of outcome, particularly lymph node status. Tumor size, another important predictor of outcome (Table 3), was only recently incorporated into the staging system. In an effort to maintain consistency and international relevancy, the FIGO system is based on limited examination methods, including palpation, inspection, colposcopy, endocervical curettage, hysteroscopy, cystoscopy, proctoscopy, intravenous pyelography, chest radiography, and skeletal radiography.18 FIGO specifically prohibits the use of surgical staging and other studies that may not be accessible to patients with limited resources. However, because regional involvement with carcinoma frequently influences prognosis and frequently alters the selection of treatment, most patients should have additional evaluation of the pelvic and abdominal lymph nodes.
Table 2. FIGO staging system for cervical cancer (1994)
Stage | |
0 | Carcinoma in situ, intraepithelial carcinoma. Cases of stage 0 should not be included in any therapeutic statistics for invasive carcinoma. |
I | The carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded). |
Ia | Invasive cancer identified only microscopically. All gross lesions, even with superficial invasion, are stage Ib cancers. |
Invasion is limited to measured stromal invasion with a maximum depth of 5 mm and no wider than 7 mm. (The depth of invasion should not be more than 5 mm taken from the base of the epithelium, either surface or glandular, from which it originates. Vascular space involvement, either venous or lymphatic, should not alter the staging.) | |
Ia1 | Measured invasion of stroma no greater than 3 mm in depth and no wider than 7 mm. |
Ia2 | Measured invasion of stroma greater than 3 mm and no greater than 5 mm in depth and no wider than 7 mm. |
Ib | Clinical lesions confined to the cervix or preclinical lesions greater than Ia. |
Ib1 | Clinical lesions no greater than 4 cm in size. |
Ib2 | Clinical lesions greater than 4 cm in size. |
II | The carcinoma extends beyond the cervix, but has not extended to the pelvic wall; the carcinoma involves the vagina, but not as far as the lower third. |
IIa | No obvious parametrial involvement. |
IIb | Obvious parametrial involvement. |
III | The carcinoma has extended to the pelvic wall; on rectal examination there is no cancer-free space between the tumor and the pelvic wall; the tumor involves the lower third of the vagina; all cases with hydronephrosis or nonfunctioning kidney should be included unless they are known to be due to other cause. |
IIIa | No extension to the pelvic wall, but involvement of the lower third of the vagina. |
IIIb | Extension to the pelvic wall or hydronephrosis or nonfunctioning kidney. |
IV | The carcinoma has extended beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum. |
IVa | Spread of the growth to adjacent organs. |
IVb | Spread to distant organs. |
Notes to the Staging System | |
Stage 0 comprises those cases with run-thickness involvement of the epithelium with atypical cells but with no signs of invasion into the stroma. | |
As a rule, it is impossible to estimate clinically whether a cancer of the cervix has extended to the corpus. Extension to the corpus should therefore be disregarded. | |
A patient with a growth fixed to the pelvic wall by a short and indurated but not nodular parametrium should be allotted to stage IIb. It is impossible, at clinical examination, to decide whether a smooth and indurated parametrium is truly cancerous or only inflammatory. Therefore the case should be placed in stage III only if the parametrium is nodular to the pelvic wall or if the growth itself extends to the pelvic wall. | |
The presence of hydronephrosis or nonfunctioning kidney due to stenosis of the ureter by cancer permits a case to be allotted to stage III even if, according to the other findings, the case should be allotted to stage I or stage II. | |
The presence of bullous edema, as such, should not permit a case to be allotted to stage IV. Ridges and furrows into the bladder wall should be interpreted as signs of submucous involvement of the bladder if they remain fixed to the growth at polposcopy (i.e., examination from the vagina or the rectum during cystoscopy). A finding of malignant cells in cytologic washings from the urinary bladder requires further examination and biopsy from the wall of the bladder. | |
Rules for Clinical Staging: | |
The staging should be based on careful clinical examination and should be performed before any definitive therapy. It is desirable that the examination be performed by an experienced examiner under anesthesia. | |
The clinical stage must under no circumstances be changed on the basis of subsequent findings. | |
When it is doubtful to which stage a particular case should be allotted, the case must be referred to the earlier stage. | |
For staging purposes the following examination methods are permitted: palpation, inspection, colposcopy, endocervical curettage, hysteroscopy, cystoscopy, proctoscopy, intravenous urography, and x-ray examination of the lungs and skeleton. Suspected bladder or rectal involvement should be confirmed by biopsy and histologic evidence. | |
Findings by examinations such as lymphangiography, arteriography, venography, laparoscopy, etc., are of value for the planning of therapy, but because these are not yet generally available and also because the interpretation of results is variable, the findings of such studies should not be the basis for changing the clinical staging. | |
Infrequently, it happens that hysterectomy is carried out in the presence of unsuspected extensive invasive cervical carcinoma. Such cases cannot be clinically staged or included in therapeutic statistics, but it is desirable that they be reported separately. | |
Only if the rules for clinical staging are strictly observed will it be possible to compare results among clinics and by differing modes of therapy. | |
Benedet J, Odicino F, Maisonneuve P, et al: Carcinoma of the cervix uteri. J Epidemiol Biostat 3:5, 1998.
Table 3. Relation between tumor size and outcome in patients treated with radiation alone
Tumor Size (cm) | Number of Patients | Central Tumor Control Rate (%) | Pelvic Tumor Control Rate (%) | Disease-Specific Survival Rate (%) |
<5 | 701 | 99 | 98 | 90 |
5–5.9 | 200 | 93 | 85 | 69 |
6–6.9 | 99 | 92 | 79 | 69 |
7–7.9 | 55 | 90 | 81 | 58 |
≥8 | 48 | 69 | 57 | 40 |
Eifel PJ, Morris M, Wharton JJ, et al: The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 29:9, 1994.
A variety of methods is available for evaluation of lymph node status, including computed tomography (CT), magnetic resonance imaging (MRI), sonography, lymphangiography, and surgical staging. Today, CT is the most common method used to evaluate lymph nodes, although its accuracy may be poor, particularly in the para-aortic nodes.19 Some clinicians favor MRI because it provides more detailed imaging of the primary site than CT; however, MRI is no better than CT for evaluation of lymph nodes.20 In a prospective comparison, the Gynecologic Oncology Group (GOG) demonstrated that lymphangiography was a more accurate method than CT for diagnosis of aortic node metastasis, but lymphangiography is no longer available in most hospitals.19 Most recently, several studies have suggested that positron emission tomography may be more sensitive than CT in the detection of metastases from cervical cancer.21 Grigsby et al have reported a strong correlation between abnormal post-therapy FDG uptake and tumor recurrence.22 Inconsistent reimbursement for the study continues to be a barrier to widespread use in United States and the cost of the study currently prevents its widespread international use in this generally medically-underserved group of patients. No radiologic study has achieved a sufficient level of accuracy to serve as a substitute for biopsy if the information so gained would significantly influence the choice of treatment. When abnormalities are found, they can be confirmed with fine-needle biopsy.
Although these radiologic and surgical studies are often useful in selecting the best treatment for patients, they should not be used to alter the FIGO stage. Inconsistent use of such information to alter the assigned stage in conflict with FIGO rules only makes it more difficult to compare results between institutions.
Surgical staging of lymph nodes is unquestionably the most accurate staging method but is invasive and can delay treatment of the primary lesion. It should not be forgotten that even lymph node dissection does not identify all patients with nodal disease. The sensitivity of surgical lymph node evaluation depends on the nodes removed and on the surgical pathologist’s treatment of the specimen. Initial studies of treatment with high-dose extended-field radiation therapy after a transperitoneal surgical procedure yielded high complication rates (15–30%) and poor survival rates (approximately 10%) for patients with positive para-aortic nodes.23, 24 However, it appears that there is little increase in the risk of late radiation complications if surgical lymph node evaluation is performed using an extraperitoneal approach.25 More recently, the use of laparoscopic lymph node dissection has been explored by several investigators. A GOG study suggested that laparoscopic dissection does not dramatically increase the risk of late complications from subsequent radiation therapy.26, 27
In its manual for staging of cancer, the American Joint Committee on Cancer (AJCC) suggests a pathologic, TNM staging system that can be used to classify surgically treated patients.28 Although this system can be used to consistently classify surgically treated patients, the use of this system should always be clearly explained to avoid confusion between AJCC stage and FIGO clinical stage. All patients should always have a clinical stage assigned preoperatively to facilitate comparison of experiences between institutions.
TECHNICAL ASPECTS OF RADIATION THERAPY
Radiation therapy for intact carcinoma of the cervix usually involves a combination of EBRT and brachytherapy (usually ICRT). The goal of treatment is to balance EBRT and brachytherapy in a way that maximizes the likelihood of local-regional tumor control while minimizing the risk of treatment complications. For many patients with local-regionally advanced disease, these treatments must also be carefully integrated with chemotherapy to achieve the greatest likelihood of cure.
The primary goals of EBRT are to sterilize regional disease and to shrink central tumor to facilitate subsequent brachytherapy. The degree of tissue penetration achieved by a radiation beam is related to the energy of the x-rays delivered from the radiation source. One of the earliest sources used for EBRT was Co 60, which produced a relatively deeply penetrating beam. However, linear accelerators now provide higher energy photon beams (15–25 MV) that are better suited for EBRT because they permit more homogeneous delivery of radiation to deep tissues with relative sparing of superficial tissues.
In ICRT for cervical cancer, radioactive sources placed in the uterine cavity are used to deliver a very high dose to the cervix and uterus with relative sparing of surrounding tissues, such as the bladder, rectum, small bowel, and superficial soft tissues. Because the radiation dose is proportional to the square of the distance from a source of radiation, tissues close to a source will receive a much higher dose than those farther away. The therapeutic advantage of ICRT always depends on the ratio of the distance between the sources and the tumor and the distance between the sources and critical normal tissues. Radium was first used to treat uterine malignancies shortly after its discovery at the turn of the century and was convenient because of its very long half-life (Table 4). However, because the daughter product of radium—radon gas—can pose a radiation protection problem, 137Cs, which provides similar energy, has now replaced radium in many practices.
Table 4. Radioisotopes commonly used in treatment of cervical cancer
Photon Energies (MeV) | |||||
Name | Symbol | Half-Life | Range | Average | Comments |
Cobalt |
| 5.26 years | 1.17, 1.33 | Commonly used for EBRT in the past; occasionally used in HDR or LDR brachytherapy | |
Cesium |
| 30.0 years | 0.662 | Most commonly used source for LDR ICRT | |
Radium |
| 1600 years | 0.047–2.45 | 0.83* | Most commonly used source for LDR ICRT before 1980s; potential for leakage of radon gas led to gradual replacement |
Iridium |
| 74.2 days | 0.136–1.06 | 0.38 | Most commonly used source for gynecologic interstitial brachytherapy and for HDR ICRT |
EBRT, external-beam radiation therapy; ICRT, intracavitary radiation therapy; LDR, low-dose–rate; HDR, high-dose–rate
*Average γ energy with 0.5-mm platinum filtration encasing source
Khan FM: The Physics of Radiation Therapy. Baltimore, MD: Williams & Wilkins, 1994
Low-dose–rate ICRT with cesium or radium provides an additional therapeutic advantage by permitting recovery of sublethal injury to normal tissues during the course of irradiation. In more recent years, computer technology has made it possible to deliver ICRT at high dose rates (more than 100 cGy/min) using high-activity sources, usually 192Ir. These approaches are discussed in further detail in the Dose Rate section.
Radiation Therapy Planning
EBRT is used to shrink bulky endocervical tumors to bring them within the range of the high-dose portion of the ICRT dose distribution (Fig. 1), to shrink exophytic tumors that distort anatomy, and prevent optimal brachytherapy, and to sterilize disease (paracentral and nodal) that lies beyond the reach of the ICRT system.
Patients with bulky central disease usually begin treatment with a course of EBRT. Some practitioners (e.g. Perez and colleagues30) prefer to maximize the brachytherapy component of treatment and deliver the first ICRT treatment as soon as EBRT has shrunk the tumor sufficiently to permit good placement of an intracavitary system (with bulky tumors, this may still require 40 Gy or more of EBRT).30 Subsequent irradiation of the pelvic lymph nodes is done using anterior and posterior fields with a block that shields central structures that received the greatest dose from ICRT. Other radiation oncologists opt to treat most patients with large central lesions with an initial EBRT dose of 40–45 Gy to the whole pelvis.
The first approach (brachytherapy as soon as possible) delivers a greater proportion of the central dose with brachytherapy and may reduce the volume of bladder and rectum treated to a high dose; however, with this approach, more reliance is placed on the extremely complex match between the three-dimensional dose distribution from the ICRT system and the edge of the central block in the external fields. Initial treatment of the whole pelvis to a total dose of 40–45 Gy provides a homogeneous distribution to the entire region at risk for microscopic disease and may produce somewhat more shrinkage of central disease before ICRT. In fact, both approaches have been in use for several decades and, when optimally employed, appear to yield excellent tumor control rates with acceptable complication rates. However, it is probably best not to exceed a radiation dose of 40–45 Gy to the whole pelvis because higher doses have been associated with higher complication rates without improvement in pelvic disease control.31
Patients who have only microinvasive disease are most often treated with simple hysterectomy. However, medical problems or morbid obesity may complicate surgery, and in such cases, ICRT alone yields good results. Some patients with very small stage IB disease (less than 1 cm) may also be treated with ICRT alone, particularly if there are relative contraindications to a course of EBRT.32 Patients who have relatively small lesions and a narrow vagina should be treated with ICRT first to optimize the brachytherapy portion of treatment before EBRT causes additional narrowing of the vaginal apex. A total dose (EBRT plus ICRT) of 50–55 Gy appears to be sufficient to sterilize microscopic disease in the pelvic nodes in most patients. Nodes known to contain gross disease and heavily involved parametria should be treated with additional EBRT with a small boost field to a total dose of 60–65 Gy.
External Beam Radiation Therapy Technique
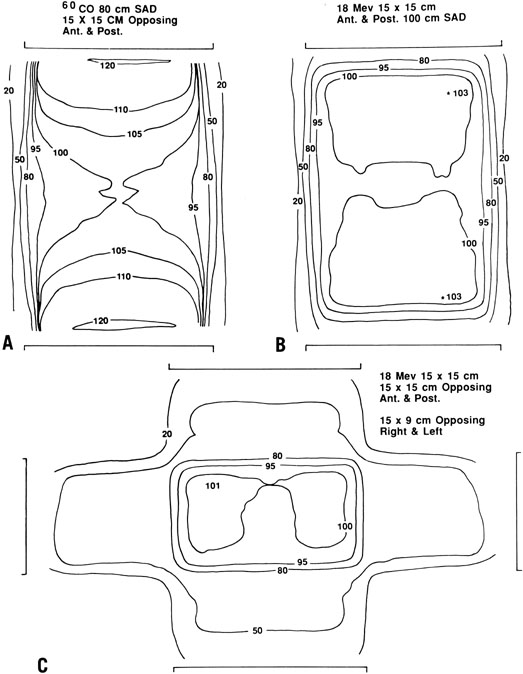
For most patients with cancer of the cervix, relatively high beam energies (15–25 MV) are preferred because they spare superficial tissues that are not usually at risk for disease. When lower energy beams (e.g. Co 60 or 6–10 MV) are used, multiple-field techniques (usually anterior, posterior, and lateral fields) should be used to reduce the dose to subcutaneous tissues (Fig. 2). Treatment technique and custom blocking must be carefully tailored to encompass the patient’s known disease and potential sites of microscopic disease. Today, treatment is usually planned using CT or MRI scans that may help the radiation oncologist define a target volume. However, during treatment planning, it is important to take into account that the position of the cervix can move by as much as 3–4 cm with changes in bladder filling.
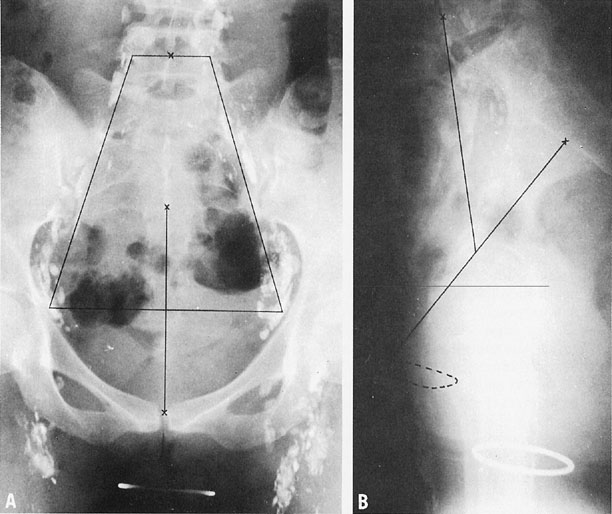
To treat the whole pelvis, treatment fields typically extend from the L4-5 interspace superiorly to the midpubis or to a line 4 cm below the lowest vaginal disease (Fig. 3). Lateral borders are placed at least 1 cm lateral to the pelvic margins. The lateral margins and shielding should be more generous if massive obesity reduces the reproducibility of the treatment setup.
If a four-field technique is used, the anterior border of the field usually includes the anterior tip of the pubis; to cover the tumor, presacral nodes, and uterosacral ligaments, the posterior border usually includes S3 (see Fig. 3). Custom blocks may be used to shield anterior small bowel, soft tissue, and, in some cases, low rectum on the lateral fields. However, care must be taken not to shield potential sites of disease. Because the inguinal nodes drain the lower third of the vagina, they should be included in the treatment volume whenever the distal vagina is involved with tumor.
The risk of major complications is increased when the dose of whole-pelvic radiation exceeds 40 Gy at 2 Gy per fraction or 45 Gy at 1.8 Gy per fraction.31 There is no clear evidence that fraction sizes of less than 2 Gy significantly decrease the rate of late complications, although daily fractions of 1.8 Gy may reduce the severity of acute radiation effects when large treatment fields or concurrent chemotherapy is used. Although 40–45 Gy is usually sufficient to control microscopic tumor in the pelvis, additional treatment must be given to control gross disease. ICRT is used to deliver a high dose to cancer in the cervix; enlarged pelvic nodes or lateral parametrial tumor may lie beyond the high-dose range of ICRT but may be given additional treatment with small external-beam fields.
Whole-pelvis EBRT fields typically are designed with an upper border placed either at the L4-5 interspace. However, the fields may be extended superiorly if the para-aortic nodes are known to be involved or are believed to be at high risk for involvement. Because cervical cancer typically follows an orderly progression along the lymph node chain, the upper border is selected by balancing an estimate of the risk of disease at a given level against the expected morbidity from large-volume EBRT. In general, the upper border is placed at least 4–6 cm above known disease. This is probably sufficient if the patient has had a lymph node dissection with negative nodes above the level of involvement. Bulky or multiple nodes probably warrant greater extension of the fields, particularly if the patient has not had surgical evaluation of the nodes. Although the survival rate of patients with aortic node metastases is significantly less than that of patients with similar-stage disease who do not have this finding, about 20–40% of patients with aortic node metastases are curable with radiation alone, depending on the extent of pelvic disease (Table 5).
Table 5. Survival of patients with biopsy-proven para-aortic node involvement after extended-field radiation therapy
Study | Number of Patients | % of Patients with FIGO Stage I or II Tumors | 5-Year Survival Rate (%) |
Piver et al:24 | 31 | 35 | 10 |
Berman et al:33 | 98 | 65 | 25* |
Podczaki et al:34 | 33 | 70 | 31 |
Ballon et al:35 | 18 | 78 | 23 |
Kim et al:36 | 43 | 79 | 24 |
Brookland et al:37 | 15 | 80 | 40* |
Komaki et al:38 | 15 | 93 | 40 |
Cunningham et al:39 | 2 | 100 | 48 |
Rubin et al:40 | 14 | 100 | 50 |
* Three-year survival rate
In the late 1980s, two randomized trials evaluated the role of prophylactic extended-field irradiation in patients with locally advanced cervical cancer.41, 42 Patients who had known clinical evidence of aortic node involvement were ineligible for these trials. The Radiation Therapy Oncology Group (RTOG) 79-2042 compared pelvic irradiation with extended-field (upper border L1-L2) radiation therapy in patients with stage I or II disease. No routine surgical or radiographic evaluation of the aortic lymph nodes was performed. Analysis of this trial demonstrated a significantly better survival rate for patients who received prophylactic aortic irradiation in addition to pelvic irradiation and ICRT. A second trial, performed by the European Organization for Research and Treatment of Cancer (EORTC),41 involved a similar randomization but had more extensive clinical staging of the abdomen (patients were required to have negative para-aortic nodes as determined by lymphangiography) and included patients with more advanced disease (bulky stage II or stage III). The EORTC trial failed to demonstrate a significant improvement with extended fields. The locally advanced tumors included in this trial and the corresponding high local failure rate may have reduced the potential benefit of large-field irradiation. The relative benefit of prophylactic extended-field irradiation may also be reduced when more accurate evaluations of regional metastases are available. Today, prophylactic aortic irradiation is performed infrequently because a later RTOG trial6 found an even greater advantage from pelvic irradiation and concurrent chemotherapy.
Although EBRT plays an important role in the treatment of cervical cancer, the high central dose delivered with ICRT is also an extremely important component of curative treatment of cervical cancer. Patients should be treated with EBRT alone only in the rare case of massive, poorly responsive disease in which the uterine canal cannot be probed, even with ultrasound guidance. In our experience, this represents fewer than 1% of cases. In other cases, locally advanced disease is better treated with a combination of EBRT to the whole pelvis, ICRT, and carefully planned parametrial or nodal boosts.31
Brachytherapy Technique
Intracavitary Radiation Therapy
Intracavitary Applicator Systems: a number of different intracavitary applicator systems have been developed to treat cervical cancer. Nearly all include an intrauterine tube and some form of vaginal applicator to accommodate radiation sources; the length of the intrauterine tube (tandem) and the design of the vaginal applicators vary between systems.
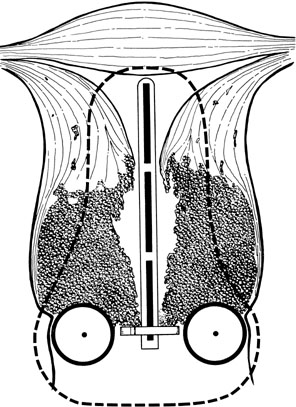
The Fletcher-Suit-Delclos applicator used at The University of Texas MD Anderson Cancer Center43, 44 consists of a rigid metal tandem with an adjustable flange that can be set to correspond to the length of the uterine cavity and two cylindrical colpostats that are positioned in the vaginal fornices (Fig. 4). The applicator is loaded with radium or cesium in the patient’s room after the accuracy of placement has been verified radiographically and the patient has recovered from anesthesia. Remote afterloading units are now available with Fletcher-Suit-Delclos–type applicators. These systems reduce the radiation exposure to personnel to a negligible level.
Recommendations for loading of Fletcher-Suit-Delclos applicators should not be generalized to other types of applicators because differences in the dose distribution around the colpostats can result in substantial differences in the doses delivered to the mucosa of the upper vagina, base of the bladder, and anterior rectal wall.
Placement of Intracavitary Systems
Delclos and associates44 described the following conditions for successful ICRT:
- The geometry of the radium sources must prevent underdosed regions on and around the cervix.
- An adequate dose must be delivered to the para-cervical areas.
- Mucosal tolerance must be respected.
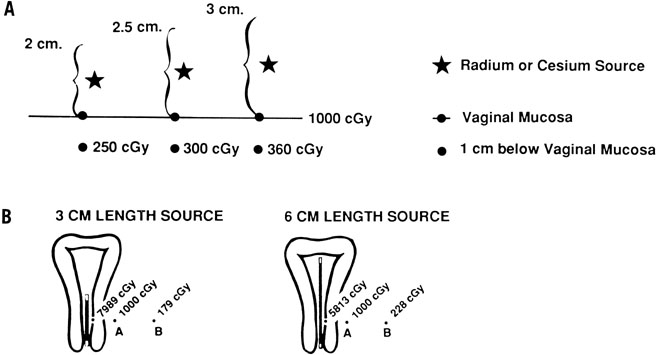
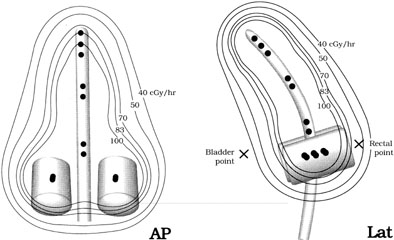
The physics of ICRT depend on the inverse square law. Fig. 5 illustrates the influence of the inverse square law on the depth dose from colpostats and uterine tandems. The radiation dose to a point in the pelvis depends on the amount of cesium in each source, the distance from each source to the reference point, and the length of time the radioactive sources remain in place. Optimal placement of the uterine tandem and vaginal ovoids produces a pear-shaped distribution laterally, delivering a high dose to the cervix and paracervical tissues and a reduced dose to the rectum and bladder (Fig. 6).
The manipulations necessary to optimize placement of the intracavitary system in different anatomic situations can only be learned with experience. To maximize the chance of local control in the treatment of bulky cervical tumors, treatment is prescribed to deliver the highest dose to the tumor that will not produce an unacceptable risk of major complications. Because there may be a narrow margin between tumor control and normal tissue complications, the radiation oncologist must make every effort to optimize the intracavitary system to obtain a favorable ratio between the dose to the tumor and the dose to adjacent normal tissues. To maintain an acceptable dose rate to bladder and rectum, the axis of the tandem is usually placed in a midposition in the pelvis and is loaded with approximately 5–6 mgRaEq of 137Cs per centimeter. A relatively long line of intrauterine sources delivers a higher dose to the endocervix and to paracervical tissues; if anatomy permits, 6–7 cm is usually loaded with active sources. The vaginal ovoids are placed up against the cervix, centered on the cervical portio; on a lateral radiograph, this usually means that the colpostats are bisected by the tandem. The vagina is packed to displace the rectum and bladder away from the intravaginal sources and to keep the system in place. Colpostats of different sizes are selected as accommodated by the patient’s vaginal anatomy and are usually loaded with 10–20 mgRaEq of 137Cs according to the size of the colpostats; typical loadings usually produce a dose rate of 80–100 cGy per hour at the lateral surface of the vagina.
Timing
Therapy usually is accomplished in two equal applications given approximately 2 weeks apart. For patients who have received initial EBRT, the first intracavitary placement should be administered as soon as possible after completion of EBRT (usually 1–5 days). In most cases, the entire course of treatment should be completed in less than 8 weeks. Excessive protraction of the overall treatment time will compromise its effectiveness.45, 46, 47
Treatment Prescription and Dose Specification
Methods of ICRT treatment prescription and dose specification have generally been developed empirically and differ significantly, making it difficult to compare experiences between institutions. There is no inherently correct way to specify the inhomogeneous radiation dose distribution delivered with an intracavitary system. The most common method of paracentral specification has been to describe the dose at a single reference point, usually one located 2 cm lateral to and 2 cm superior to the external cervical os in the plane of the intrauterine tandem (point A). Point A is a reference point developed as part of the Manchester system, which also incorporated a set of fairly rigid rules guiding application geometry and radioactive source loadings. Point A bears no consistent relation to tumor volume or target volume and has been localized in different ways by different investigators. In 1985, the International Commission on Radiation Units and Measurements48 recommended that reference points like point A not be used because “such points are located in a region where the dose gradient is high and any inaccuracy in the determination of distance results in large uncertainties in the absorbed doses evaluated at these points”. Instead, it was recommended that doses be specified in terms of (1) total reference air Kerma (TRAK), which is expressed in milligray at 1 meter and gives information similar to that described by the “concept of mg-hr”; (2) description of the reference volume, that is, the tissue volume encompassed by a reference isodose surface; and (3) the dose calculated to specific reference points associated with normal tissues within the treatment volume (bladder, rectum, and vagina).
Despite these recommendations, point A is still the most common method used to specify intracavitary implants in the treatment of cervical cancer. However, dose specification for the purpose of treatment comparison should never be confused with dose prescription. Treatment should always be prescribed after careful consideration of information about the quality of the application, size of the applicator system, position of the system in the pelvis, exposure to normal tissue structures, initial tumor extent, and residual tumor, as well as the dose to tumor and normal tissue reference points.
Treatment should also take into account the dose to lateral and more distant pelvic structures. With ICRT, the dose to lateral structures is maximized when a relatively long tandem and large colpostats are used because a broader distribution of sources and greater displacement of the vagina permit more activity to be used without exceeding the maximum tolerable dose to bladder or rectum. However, care must be taken not to exceed a tolerable dose to the whole pelvis. The total mg-hrs of radium (the product of the total activity of 226Ra sources and the duration of treatment) has been used to limit the integral dose to pelvic structures; clinicians found that exposures of more than 6500 mg-hr after 40 Gy of EBRT tended to cause excessive pelvic fibrosis and bowel damage even when the doses to bladder and rectum were within acceptable limits.49 Although mgRaEq hours are often specified in a similar fashion when 127Cs is used to treat cervical cancers, care must be taken to correct for differences in the filtration of material used to encapsulate radium and cesium sources; to avoid confusion, TRAK dose is a preferred measure. A variety of reference points also have been used to estimate the dose to lateral pelvic structures. Point B (in the Manchester system) is usually located 3 cm lateral to point A, but its location in the pelvis varies considerably according to the position of the intracavitary system. Some clinicians also record the dose at a pelvic reference point P located just inside the lateral pelvic wall at the approximate level of the obturator lymph nodes.50 In the Fletcher system, bony landmarks are used to estimate the locations of the iliac lymph nodes (Fig. 7). The external iliac nodes generally lie in a plane that intersects the tip of the pubic symphysis and the middle of S2. The common iliac nodes lie along a plane between the anterior aspect of L4 and the bisector of the line between the pubis and S2. Most clinicians also calculate the dose of radiation at reference points on the lateral vaginal wall, posterior bladder wall (usually on the posterior border of a contrast-filled Foley bulb), and anterior rectal wall as viewed on orthogonal radiographs of the pelvis.48 In most cases, the doses to reference points on the vaginal surface are kept within a range of 110–130 Gy; the doses to the bladder and rectal reference points are usually less than 75 Gy and 70 Gy, respectively. However, CT-based three-dimensional analyses of the doses delivered to normal structures from ICRT demonstrate that standard reference points routinely underestimate the maximum dose delivered to these structures.51, 52 The artifact caused by standard intracavitary systems and other logistical concerns have made it difficult to perform routine three-dimensional analysis of treatment doses, but this is expected to be an active area of research in the next decade.
With typical sources loaded in a Fletcher-Suit-Delclos intracavitary system, the dose-rate at point A is usually 45–50 cGy per hour. Typically patients who have received 40–45 Gy to the pelvis are treated with two 48-hour applications, yielding a total dose to point A (with EBRT and ICRT) of 85–90 Gy for locally advanced tumors or 80–85 Gy for small IB1 tumors. However, these doses may vary depending on the clinical situation and other factors discussed above.
Dose Rate
Until relatively recently, most ICRT was delivered at a low dose rate of 40–60 cGy per hour. This was necessitated by practical considerations: the use of relatively low activity sources limited exposure of personnel to ionizing radiation during loading and unloading of the applicators and during nursing visits. However, laboratory and clinical studies also demonstrated that radiation therapy delivered at these low dose rates has an important radiologic advantage.53 The protracted continuous exposure has an effect similar to that of fractionated EBRT. The repair of sublethal injury in normal tissue cells exceeds that of tumor cells, maximizing the therapeutic ratio of ICRT.
The development of computer-driven remote afterloading devices has made it possible to deliver brachytherapy with high activity sources with negligible exposure to personnel. Using these devices, radiation is delivered by advancing a single high-activity source along a track from the machine through the brachytherapy applicator. The length of time the source rests in various positions determines the distribution of radiation dose. This form of high-dose–rate brachytherapy is now used for a number of applications, including postoperative treatment of the vaginal apex, and in some practices, treatment of intact cervical cancers. However, because the dose-rate effect is lost, treatment must be divided into multiple fractions to achieve an acceptable ratio between the rates of tumor control and late radiation complications. Clinicians still disagree about the number of fractions needed to achieve optimal results; the number of high-dose–rate treatments used to treat cervical cancers in different centers ranges from 3–15.54, 55, 56
Over the past 10–20 years, there has been a gradual increase in the use of high-dose–rate ICRT to treat intact carcinoma of the cervix in the United States. In a random survey of radiation therapy practices in the United States, 9% of patients treated between 1992 and 1994 were treated with this method.55 High-dose–rate ICRT is the dominant method of brachytherapy treatment in a number of other countries, including Japan and Germany. Despite the possible radiobiologic concerns, high-dose–rate ICRT has a number of advantages. Treatment does not require hospitalization. Although more applications are needed than with low-dose–rate treatment, the ability to give all treatment on an outpatient basis is frequently more convenient for patients and their physicians. Personnel exposure to ionizing radiation is virtually eliminated, and shielded inpatient hospital rooms are not required. For physicians who treat relatively few cases, capital costs may be less because the treatment can be delivered with the same high-dose–rate unit that is used for other purposes, eliminating the need to purchase cesium (which can be used only for cases of cervical or inoperable endometrial cancer). It has also been argued that the retraction methods used for high-dose–rate therapy, fixation of the applicator position during planning and treatment, and computerized optimization of source dwell positions may produce a more favorable ratio between the dose to tumor and normal tissues. Advocates believe that these factors overcome the radiobiologic disadvantages of treatment with large fractionated doses of radiation.
However, we still prefer the use of low-dose–rate ICRT when possible, particularly for patients who have large tumors and poorly distensible vaginas. For such patients, the maximum dose to normal tissues may be as high as or higher than the dose to tumor. In such circumstances, low-dose–rate ICRT would be expected to have an important advantage over high-dose–rate therapy. Unfortunately, no well-designed randomized study has compared the two approaches; the large number of patients that would be required, the expense of such a trial, and the unwillingness of most practitioners to maintain the equipment and sources needed to practice both low-dose–rate and high-dose–rate therapy make it unlikely that such a study will ever be done. Although retrospective studies of patients treated for cervical cancer suggest that good survival rates can be achieved with high-dose–rate treatment, poor follow-up, small numbers of patients, and selection biases compromise many of these studies. The largest United States experience reported to date still included fewer than 200 patients with all stages of disease; in that study, patients with stage III disease who were treated with high-dose-rate ICRT had a significantly poorer outcome than those in a historic comparison group treated with low-dose–rate therapy, but within the limits of statistical power, patients with earlier disease appeared to have similar outcomes with the two approaches.57
Interstitial brachytherapy
Although ICRT is the most important method of brachytherapy used for treatment of cervical cancer, some tumors, particularly those that involve the distal vagina, may be difficult to treat adequately with ICRT alone. In a small percentage of these cases, distal vaginal lesions may require treatment with a supplemental dose of radiation delivered using intralesional 192Ir interstitial implants. Some clinicians have advocated broader use of interstitial therapy as an alternative to ICRT for treatment of locally advanced cervical cancers.58, 59 With this approach, needles are inserted transperineally into the cervix and paracervical tissues; a Lucite template is used to guide and maintain parallel positioning of the needles. With this technique, the dose to paracervical tissues is usually somewhat higher than that delivered with ICRT, but the central dose to the cervix is usually somewhat lower. Thus far, most published series of interstitial brachytherapy as an alternative to ICRT have included fewer than 75 patients, and results have not been demonstrably better than those achieved with ICRT in patients with similar-stage disease.60, 61 However, this approach can be very useful for occasional recurrent cancers or intact cervical cancers that cannot be treated with ICRT.
ROLE OF CHEMOTHERAPY IN COMBINATION WITH RADIATION THERAPY
Our understanding of the role of chemotherapy in the treatment of locally advanced cervical cancer has changed dramatically in the last 5 years. As recently as 1996, in a consensus conference sponsored by the National Institutes of Health, it was concluded: “The optimal role for chemotherapy in the treatment of early or advanced invasive cervical cancer is unknown. A number of clinical trials have been completed or are underway, but at this time there is no proven benefit to combining chemotherapy with radiation”. Three years later, the first three6, 7, 8 of six large randomized trials investigating the role of concurrent cisplatin-based chemotherapy with radiation therapy were published in the New England Journal of Medicine. The results were noted in a National Institutes of Health clinical alert and have significantly changed the standard of care for many patients with local-regionally advanced cervical cancer.
A number of chemotherapeutic agents have modest activity against metastatic or recurrent carcinoma of the cervix. Cisplatin is probably the most active single agent, with response rates of 20–40%.62 Complete responses of advanced and recurrent disease are rare and generally of short duration. Higher overall response rates (usually 50–60%) have been reported in patients with newly diagnosed local-regionally advanced cancers treated with multiagent induction chemotherapy (Table 6).63, 64, 65, 66, 67, 68 Despite this, randomized trials have repeatedly failed to demonstrate a significant survival advantage when radiation therapy is preceded by chemotherapy. In two of the randomized trials, the survival rate was actually poorer for patients treated with neoadjuvant chemotherapy, despite encouraging response rates to the induction regimens.66, 68 Although the side effects of chemotherapy and diminished compliance with primary treatment recommendations may have contributed to these disappointing results, laboratory evidence suggests that accelerated repopulation of chemotherapy-resistant clones may also contribute.69
Table 6. Prospective randomized trials that investigated the role of concurrent radiation therapy and cisplatin-containing chemotherapy for patients with local-regionally advanced cervical cancer
Authors (Trial Group) | Eligibility | Number of Patients | CT in Investigational Arm | CT in Control Arm | Relative Risk of Recurrence (95% C.I.) | P |
Chemoradiation Only | ||||||
Whitney et al. (GOG)8 | FIGO IIB–IVA | 368 | Cisplatin 50 mg/m2 5-FU 4 gm2/96 hr (2 cycles) | HU 3 g/m2 (2 × /week) | 0.79 (0.62–0.99) | 0.03 |
Rose et al. (GOG)7 | FIGO IIB–IVA | 526 | Cisplatin 40 mg/m2/wk (up to 6 cycles) | HU 3g/m2 (2 × /week) | 0.57 (0.42–0.78) | <0.001 |
Cisplatin 50 mg/m2 5-FU 4 g/m2/96 hrs HU 2 g/m2 (2 × /week) (2 cycles) | HU 3 g/m2 (2 × /week) | 0.55 (0.40–0.75) | <0.001 | |||
Eifel et al 70 | FIGO IB–IIA (≥ 5 cm), IIB–IVA or pelvic nodes involved | 403 | Cisplatin 75 mg/m2 5-FU 4 g/m2/96 hr (3 cycles) | None* | 0.51 (0.36–0.66) | <0.001 |
Pearcey et al. (NCIC) ∥71 | FIGO IB–IIA (≥ 5 cm), IIB–IVA or pelvic nodes involved | 259 | Cisplatin 40 mg/m2/wk (up to 6 cycles) | None | 0.91 (0.62–1.35)†† | 0.43 |
Chemoradiation Plus Surgery | ||||||
Stehman et al 72 | FIGO IB (≥ 4 cm) | 369 | Cisplatin 40 mg/m2/wk (up to 6 cycles) | None† | 0.61 (0.43–0.85) | <0.004 |
Peters III et al. (SWOG) §9 | FIGO I–IIA after radical hysterectomy with nodes, margins, or parametrium positive | 268 | Cisplatin 70 mg/m2 5-FU 4 g/m2/96 hr (2 cycles) | None | 0.50 (0.29–0.84) | 0.01 |
CI, confidence interval; CT, chemotherapy; FIGO, International Federation of Gynecology and Obstetrics; GOG, Gynecologic Oncology
Group; HU, hydroxyurea; NCIC, National Cancer Institute of Canada; PA, para-aortic; RT, radiation therapy; SWOG, Southwest Oncology Group.
* Patients in control arm had prophylactic para-aortic irradiation
† All patients had extrafascial hysterectomy after radiation therapy
††Survival
§Chemotherapy was begun on day 1 and continued every 4 weeks through and after radiation therapy
¶Patients were also randomly assigned to receive standard or hyperfractionated radiation therapy in a four-arm trial.
∥In investigation arms. In no case was the median duration of RT significantly different between control and investigational arms.
In contrast, studies that combine radiation therapy with concurrent chemotherapy have been more encouraging. Early GOG studies emphasized the use of concurrent hydroxyurea with radiation therapy. Although small randomized studies suggested that there may have been a benefit with this approach,29, 73 flaws in trial design, drug toxicity, and, most important, the demonstrated superiority of other regimens have led most clinicians to abandon the use of hydroxyurea.
Subsequently, the GOG,5, 7, 8 the Southwest Oncology Group (SWOG),9 the RTOG,6 and the National Cancer Institute of Canada (NCIC)71 launched six trials that compared cisplatin-containing concurrent chemoradiation regimens with non-cisplatin–containing regimens in patients with various stages of local-regionally advanced cervical cancer. Two of the GOG trials included concurrent hydroxyurea in their control arms, reflecting their earlier experiences with the drug. The RTOG trial compared extended-field irradiation with a combination of pelvic irradiation, cisplatin, and 5-FU. Extended-field radiation therapy (including the pelvic and aortic nodes) had been found to be superior to pelvic irradiation in their earlier trial; extended-field radiation therapy was not used in their chemotherapy arm because of unacceptable toxicity observed with this combination in earlier phase II studies. A third GOG trial and the SWOG trial compared combinations of chemoradiation and hysterectomy with radiation therapy and surgery alone. The NCIC trial was a simple comparison of pelvic radiation therapy with or without concurrent cisplatin. The eligibility criteria and treatment arms of these trials are outlined in Table 7.
Table 7. Results of prospective randomized trials that compared neoadjuvant chemotherapy followed by radiation therapy with radiation therapy alone in patients with locally advanced cervical cancer
Survival Rate | ||||||||
Author | Year | No. Pts. | Stages | Drugs | CT + RT | RT Alone | p | End Point |
Kumar et al.64 | 1994 | 184 | IIB–IVA | BIP | 38% | 43% | .5 | OS at 32 m |
Tattersall et al.67 | 1992 | 71 | IIB–IVA | PVB | 44%* | 40%* | ns | OS at 48 m |
Chauvergne et al.63 | 1990 | 107 | IIIB | MtxCVP | 47%* | 50%* | ns | OS at 48 m |
Tattersall et al.68 | 1995 | 260 | IIB–IVA | EpP | 50%* | 69%* | .02 | OS at 36 m |
Sundfør et al.74 | 1996 | 94 | IIIB–IVA | PF | 34%* | 37%* | .9 | OS at 48 m |
Leborgne et al.65 | 1997 | 96 | IB2–IVA | BOP | 38% | 45% | .4 | DFS at 60 m |
Souhami et al.66 | 1991 | 107 | IIIB | BOMP | 23% | 39% | .02 | OS at 60 m |
B, bleomycin; C, chlorambucil; CT, chemotherapy; DFS, disease-free survival; Ep, Epirubicin; F, 5- fluorouracil; I, ifosfamide; M, mitomycin C; Mtx, methotrexate; O, vincristine; OS, overall survival; P, cisplatinum; RT, radiation therapy; V, vinblastine.
*Percentages were estimated from survival curves.
Eifel PJ, Berek JS, Thigpen JT: Cancer of the cervix, vagina, and vulva. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice, pp. 1526–1572. Philadelphia: Lippincott Williams & Wilkins, 1999.
Initial results of five of these studies (all but the NCIC trial) became available almost simultaneously in 1999 and early 2000. The results are summarized in Table 7. Although the trials differed in their eligibility and control arms, all five demonstrated significantly better survival rates in their cisplatin-containing arms, with reductions in the risks of local recurrence, distant metastasis, and death of up to 50%. The most successful regimens used in these trials were the regimen recommended by the GOG (40 mg/m2 of cisplatin weekly) and the regimen used in the SWOG and RTOG trials (cisplatin 70–75 mg/m2 plus 5-FU, 4 g/m2 over 96 hours given every 3 weeks). The GOG also tested a combination of cisplatin (at a dose of 50 mg/m2) and 5-FU; this regimen was also more effective than hydroxyurea but seemed to produce a less dramatic reduction in the risk of recurrence than other regimens. A third GOG regimen that combined cisplatin, 5-FU, and hydroxyurea was no more effective and was more toxic than weekly cisplatin.
The sixth trial, from the NCIC, was published in 2002.71 This trial used the same regimen of weekly cisplatin as the GOG trials, but unlike the other five studies, the NCIC trial failed to demonstrate an advantage with concurrent cisplatin. The reason for this difference is not obvious. The authors of the NCIC trial argue that the less protracted radiation therapy regimen used for their patients may have been more effective, obviating chemotherapy. However, an examination of the outcome of their control arm suggests that there was a similar margin for improvement in local control and in other outcome measures. It is also possible that the failure to demonstrate a significant improvement was a statistical phenomenon: with 259 patients randomized, the NCIC study was the smallest of the trials and had broad confidence intervals on the estimates of outcome.
Several other drug combinations have been tried with apparent success. Wong and colleagues75 conducted a randomized trial of radiation therapy with or without concurrent and adjuvant epirubicin (an anthracycline unavailable in the United States). Progression-free survival was significantly better for patients who received chemotherapy (p = .03). In conjunction with Yale University, a group in Venezuela has been studying concurrent mitomycin C and 5-FU, a combination that has been highly successful in patients with anal cancer. The authors have reported preliminary results that they considered to be encouraging, but the study is not yet mature and continues to accrue patients. In 1998, Thomas and colleagues76 reported the results of a four-arm trial that evaluated the role of concurrent continuous-infusion 5-FU (a successful regimen in patients with gastrointestinal adenocarcinomas). This relatively small study did not show a significant improvement in survival with 5-FU, but the results were felt to be encouraging, particularly for the subset of patients with stage I and II disease. This result stimulated a GOG comparison between weekly cisplatin and continuous-infusion 5-FU, however results from this trial showed no advantage of 5-FU over weekly cisplatin.77
Although concurrent chemotherapy is now considered to be standard treatment for many patients with locally advanced cervical cancer who require radiation therapy, the studies published to date leave numerous unanswered questions. No significant increase in late complications was detected in the early analyses, but acute side effects were more severe with concurrent chemotherapy. The advantages of concurrent chemotherapy undoubtedly outweigh the side effects for most patients. However, it should be remembered that many patients are curable with radiation therapy alone; it would desirable to be able to determine which tumors require chemoradiation and which could be treated effectively with fewer side effects using radiation therapy alone. An update of the SWOG trial78 by Monk et al tried to look retrospectively at both histopathologic and clinical factors which may predict for recurrence. This study was limited due to its retrospective nature, but they did find that the prognostic significance of histologic type, tumor size, number of positive nodes and parametrial extension in the radiation group was less apparent when chemotherapy was added. They concluded that patients with tumors less than 2 cm and one positive node may possibly be treated with radiation therapy alone. Also, a study comparing cisplatin and 5-FU with weekly cisplatin would be desirable. Although clinicians tend to prefer to avoid the trouble of delivering continuous-infusion 5-FU, oral substitutes may provide an effective solution. Investigators are also interested in exploring other drugs that might add to the success of cisplatin. However, care must be taken not to compromise the dose and effectiveness of the one drug that has most convincingly been demonstrated to be of benefit.
Another question that remains is the safety and benefit of concurrent chemotherapy in patients with positive para-aortic nodes that are receiving extended field radiation therapy. In several phase II studies, concurrent chemotherapy has been given with extended-field irradiation. Although side effects are greater when treatment fields are enlarged, combined therapy may be tolerable if careful consideration is given to the chemotherapy regimen, volume of tissue irradiated, and other factors that might increase the risk of serious toxicity. Intensity modulated radiation therapy may help in reducing both acute and late toxicity with extended field radiation therapy, and it may allow better control of bulky nodal disease by allowing higher doses to be delivered to gross disease while minimizing dose to normal tissues.
COMPLICATIONS OF RADIATION THERAPY
Complications of radiation therapy are usually characterized as acute (occurring during or shortly after treatment), or late (occurring more than a few weeks and sometimes many years after radiation therapy). The acute complications of radiation therapy usually resolve within 2–3 weeks after completion of treatment.
Pelvic irradiation may cause acute proctosigmoiditis. Two to three weeks after the beginning of treatment, patients may develop diarrhea; on rare occasions, this may be associated with passage of blood and mucus or tenesmus. These symptoms usually subside shortly after the completion of EBRT. A small proportion of patients develop chronic diarrhea that may be associated with bleeding and anemia. In such cases, flexible sigmoidoscopy reveals a smooth pale mucosa with prominent friable blood vessels. The symptoms usually subside within a few months, although rarely patients develop chronic proctosigmoiditis that may lead to stricture or obstruction. In less than 5% of patients, radiation injury to the rectosigmoid may result in progressive ischemia leading to necrosis and occasionally stricture or fistula formation. When this occurs, patients should be carefully evaluated to exclude the possibility of recurrent disease. Patients who develop a radiation-induced rectovaginal fistula require a diverting colostomy. If there is no evidence of recurrent disease and the area of necrosis does not spread, selected patients may undergo resection of the involved area with a low reanastomosis of the sigmoid to the anus. However, the rate of anastomotic failure is high after this type of repair. Necrosis above the peritoneal reflection is rare but can be catastrophic if it leads to perforation with peritonitis or abscess formation.79 Early symptoms of this serious complication include abdominal pain, vomiting, bloody diarrhea, and weight loss and should be pursued aggressively.
The acute reaction of the small intestine to radiation therapy may also cause diarrhea and abdominal cramping. Nausea and vomiting are rare unless extended fields are used. These symptoms are usually well controlled with antidiarrheal medications and antiemetics and generally disappear within a few days of the completion of radiation. Diarrhea may also respond to a low-residue diet. Patients with a history of lactase deficiency may have particularly severe symptoms and may benefit from a lactose-free diet. Chronic, symptomatic small bowel injury rarely develops unless patients have a predisposing factor. Patients who have had extensive abdominopelvic surgery and those with a history of pelvic infection are particularly prone to develop small bowel injury following irradiation. Patients who are treated with extended fields or who received particularly high doses of pelvic radiation therapy are also more likely to suffer small bowel complications.79 The terminal ileum is most frequently involved. Patients who suffer chronic radiation-induced small bowel injury usually present 1–5 years after irradiation with symptoms of intermittent abdominal distention, cramping, nausea, weight loss, and diarrhea. The diarrhea is caused by rapid transit and malabsorption. Symptoms may wax and wane over a period of months to years. In less than 2% of patients who receive standard radiation doses, more severe injuries may develop, including focal stricture, obstruction, and, in severe cases, perforation. Recent studies have demonstrated that small bowel complications are significantly more frequent in women who smoke cigarettes, particularly those who smoke more than one pack of cigarettes per day.10
Patients who have mild to moderate symptoms of small bowel obstruction can often be managed with a low-residue diet and careful observation. Patients with more severe symptoms, especially repeated episodes of partial obstruction, should receive surgical intervention. The obstructed portion of small bowel should be resected, preserving as much small bowel as possible. Intestinal bypass procedures should be performed only when resection is not technically feasible.
Approximately 5–10% of patients treated with pelvic radiation develop symptoms of dysuria and urinary frequency during EBRT for carcinoma of the cervix. When no infection is found, these symptoms usually respond to pyridium and resolve shortly after the completion of treatment. Although late radiation complications involve the bladder less frequently than the bowel, a small proportion of women will develop symptoms of radiation cystitis one or more years after radical radiation therapy. Some women will have only one or two episodes of sterile dysuria or mild hematuria.80 Rarely, radiation cystitis can cause hemorrhage requiring emergency intervention. After cystoscopy with clot evacuation, patients may benefit from continuous inpatient irrigation of the bladder with acetic acid or potassium permanganate solution or saline using a three-way Foley catheter. Extreme care should be taken to ensure that blood clots do not obstruct the outflow tract of the catheter during irrigation. Patients who do not respond to irrigation may be helped by electrocoagulation or bladder tamponade. Rarely, patients who have persistent bleeding may require cystectomy with urinary diversion.
A variety of other rare urinary tract complications may occur. After high doses of EBRT, women may develop a contracted fibrotic bladder, leading to frequent urination and, in some cases, incontinence. Severe radiation injury may result in focal necrosis leading to vesicovaginal fistula. When this occurs, a careful search for recurrent tumor is always indicated. Fistulae caused by radiation can rarely be repaired, and urinary diversion is usually required. Although ureteral obstruction usually indicates recurrent cervical cancer, it may rarely result from localized radiation fibrosis.
During radical radiation therapy for cervical cancer, the vaginal apex and superficial cervical tissues receive a high dose of radiation. It is not uncommon to see some degree of necrosis of these tissues in the weeks after treatment. In an attempt to rule out tumor recurrence, numerous biopsies are often performed, which may aggravate the tissue damage. Treatment of cervicovaginal necrosis should be conservative. Aggressive debridement may leave gaping uncorrectable defects. We recommend cleansing of the affected area with a solution of 50% peroxide and 50% water two to three times daily. Some believe that the addition of estrogen replacement therapy can assist in the healing process. After irradiation with high doses, the vaginal apex tends to become agglutinated. Vaginal stenosis and shortening tend to be more severe in patients who had large tumors or unfavorable (i.e. narrow) vaginal anatomy prior to irradiation. Regular intercourse or use of a vaginal dilator may help to maintain patency of the vaginal canal. Systemic and local application of estrogen reduces vaginal dryness. Because irradiation invariably eliminates normal ovarian function, estrogen replacement is needed for all young women who undergo pelvic irradiation to prevent osteoporosis and other sequelae of hypoestrogenism.
Patients who have received high doses of EBRT for large infiltrating cervical tumors frequently develop dense soft tissue fibrosis and 'woody' induration of pelvic tissues. In this setting, it can be very difficult to interpret findings on subsequent pelvic examinations.
The knowledgeable delivery of modern EBRT and ICRT minimizes the risk of serious late complications of radiation therapy. Overall, the risk of major sequelae is usually less than 10% overall. Although careful technique is justified, excessive caution can result in tumor recurrence, which is the worst complication for most patients. When major complications do occur, patients should be referred whenever possible to a gynecologic oncologist who is experienced in the specialized surgical techniques that are often required in an irradiated pelvis.
ROLE OF ADJUVANT HYSTERECTOMY AFTER RADIATION THERAPY
For stage I and II cervical cancer, tumor volume strongly correlates with nodal status, pelvic control, and survival (see Table 3). The relation between tumor volume and prognosis is undoubtedly a continuous one, although investigators have generally placed artificial boundaries for the purposes of analysis. Although the rates of regional and distant metastasis increase continuously as tumor invasiveness and volume increase beyond microinvasive, central relapse after radiation alone occurs in less than 2% of patients with tumors smaller than 5 cm in diameter who are treated according to Fletcher’s guidelines.81, 82, 83 No one has demonstrated that sufficiently high central recurrence rates in such patients justifies the routine use of adjuvant surgery. Central recurrence is also rare (fewer than 5%) in patients who have stage IB2 tumors with a dominant exophytic morphology, even when the tumors are more than 5 cm in diameter (see Table 3).82 However, somewhat higher recurrence rates for bulky (6 cm or more) tumors prompted Durrance and coworkers81 to recommend the use of adjuvant extrafascial hysterectomy after radiation therapy for patients with these tumors.
Subsequent studies have not confirmed the utility of adjuvant hysterectomy. A re-examination and update of the MD Anderson experience84 suggested that some of the advantage suggested in earlier retrospective reviews may have resulted from a tendency to select patients with more unfavorable tumors (those with positive nodes or very poor responses) for treatment with radiation therapy alone. Other retrospective studies did not demonstrate a clear advantage with adjuvant hysterectomy and suggested that combined treatment may increase the rate of fistula after irradiation.85, 86 Subsequently, the GOG presented results of a randomized trial comparing radiation therapy alone with radiation therapy and extrafascial hysterectomy for patients with stage IB2 disease.87 The trial demonstrated no significant difference in survival between the two treatment arms with virtually overlapping survival curves.
The most recent GOG trial in this subset of patients compared radiation plus extrafascial hysterectomy with chemoradiation plus hysterectomy.5 Combined local treatment was chosen as the control because the results of the earlier study had not yet matured and were thought to favor combined treatment. As discussed above, the arm that included chemotherapy was superior. In fact, the local control rate after chemoradiation alone was sufficiently high in patients with bulky stage I disease that it left little room for improvement with adjuvant surgery. Today, chemoradiation alone is usually the preferred treatment for such patients, with surgery reserved for the infrequent patient with persistent central disease.
IMPORTANCE OF ANEMIA IN PATIENTS UNDERGOING RADIATION THERAPY FOR CERVICAL CANCER
Tumor hypoxia has been suggested as a potential mechanism of radiation resistance in bulky endocervical tumors. In this context, the importance of anemia as an independent prognostic factor and the need for pretreatment transfusion of anemic patients has been debated. Anxiety about acquired immune deficiency syndrome (AIDS) and hepatitis has increased some patients’ reluctance to undergo transfusion. The oxygen effect may be of less importance when fractionation permits intertreatment reoxygenation.
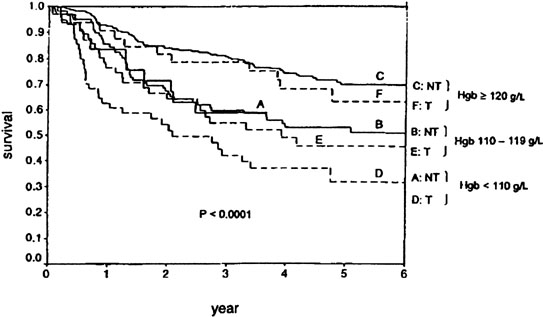
Nevertheless, hemoglobin level has clearly been a significant prognostic factor for patients with locally advanced cervical cancer. In late 1970s, in a small prospective study from Princess Margaret Hospital,88 anemic patients with stage IIB and III disease were randomly assigned to receive or not to receive transfusion. Patients with a hemoglobin level of 10–12.5 gm% who did not receive transfusions had a significantly higher local relapse rate than patients who received transfusions or those who were not anemic. Unfortunately, no attempt has ever been made to confirm this result in a larger prospective trial; however, retrospective analyses support the suggestion that transfusion can overcome the negative effects of anemia on radiation response (Fig. 8).89 Several large retrospective reviews have revealed strong, apparently independent correlations between anemia during radiation therapy and local failure.89, 90, 91 Girinski and colleagues90 found that even brief periods of severe anemia (< 10 g/mL) during radiation therapy were associated with an increased risk of local failure. A recent Gynecologic Oncology Group prospective randomized study intended to investigate the use of exogenous erythropoietin to increase hemoglobin levels was aborted after an unexpectedly high rate of thromboembolic complications was noted in early patients randomized to the erythropoietin arm and the occurrence of similar problems in studies conducted in other tumor sites. Low intratumoral Po2 levels have been shown to correlate with poor outcome after radiation therapy, but it remains unclear whether this is because of radiobiologically important intratumoral hypoxia or because hypoxia is a surrogate for other adverse tumor characteristics.92
Although some basis for criticism can be found for all of these studies, the preponderance of data strongly suggests that patients who are severely anemic (hemoglobin concentration less than 10 gm%) benefit from transfusion. Transfusion is probably indicated for patients with borderline hemoglobin levels if they continue to have bleeding. Attempts should be made to minimize bleeding during manipulative procedures and to use appropriate vaginal packing and application of Monsel’s solution. When available, transvaginal irradiation or a short course of accelerated pelvic radiation therapy may be very effective in stopping bleeding, particularly for patients with exophytic lesions.
POSTOPERATIVE RADIATION THERAPY
Radiation Therapy after Radical Hysterectomy
In a 1991 review of the results of several surgical series, 42% of the recurrences after treatment with radical hysterectomy alone for cervical cancer were limited to the pelvis; 72% involved the pelvis and other sites.93 Although clinicians disagree about the survival benefit of postoperative radiation therapy for patients with positive nodes or parametria, postoperative treatment has been clearly demonstrated to reduce the rate of pelvic recurrence;94 efforts to complete randomized comparisons of radical hysterectomy with or without radiation therapy have been unsuccessful, and patients who undergo radical hysterectomy are usually offered postoperative radiation therapy. With the recent publication of positive results of a randomized trial comparing postoperative radiation therapy with or without chemotherapy (see Table 4),9 combined chemoradiation has become the standard treatment for most patients with positive nodes or parametria or who have positive or close surgical margins.
In patients with negative pelvic nodes, deep stromal penetration (greater than 10 mm or more than 70% of the stromal thickness), large tumor diameter (4 cm or more), and lymph-vascular space invasion are predictors of pelvic recurrence.95, 96, 97, 98 Findings of a prospective GOG randomized trial (GOG 92), recently updated by Rotman et al,99 suggest that postoperative pelvic radiation therapy may benefit patients with intermediate risk features after radical hysterectomy. The authors reported a 46% reduction in the risk of recurrence (p = 0.007) and a significant improvement in recurrence or death when post-operative pelvic irradiation was given; however, there was no statistical significance in survival between the two arms.
Postoperative radiation therapy does add to the risk of major treatment complications, particularly small bowel obstruction,99, 100 and also increases the overall cost of treatment. For these reasons, patients whose pretreatment characteristics suggest a high likelihood that radiation therapy will be required after treatment with initial hysterectomy should probably be strongly considered for treatment with radiation therapy alone. A prospective randomized trial comparing radiation therapy alone versus radical hysterectomy demonstrated that more than 80% of patients with clinical stage IB2 disease (i.e. more than 4 cm diameter) had tumors that would require additional adjuvant treatment with radiation therapy or chemoradiation.101 Partly for this reason, patients who were treated with initial surgery had a higher overall complication rate than those treated with radiation therapy alone. Better methods of selecting patients for initial local treatment continue to be needed. Both MRI and positron emission tomography may provide means of improving diagnostic accuracy in the future.21, 102 Recently, the University of Chicago published data on the reduction of both acute and chronic GI side-effects in patients with both cervical and endometrial carcinoma treated in the post-hysterectomy setting with intensity modulated radiation (IMRT) compared to standard therapy.103, 104 IMRT may reduce toxicity, however it needs to be tested in a larger setting and presently the RTOG is doing a phase II trial looking at IMRT in this setting.
Radiation Therapy After Simple Hysterectomy Performed in the Presence of Unsuspected Invasive Cervical Cancer
When simple hysterectomy is inadvertently performed in the presence of invasive cervical cancer, a situation commonly referred to as cut-through hysterectomy, further treatment is needed to prevent possible recurrence in the parametria, vagina, and pelvic nodes. This continues to be a significant clinical problem despite the widespread use of cervical screening and education of gynecologists in the technique of colposcopy. In a 1992 review, Roman and coworkers105 noted that the most common reasons for inappropriate hysterectomy were false-negative findings on Pap smear, failure to properly evacuate an abnormal Pap smear, and failure to perform an indicated conization of the cervix.
Patients who have undergone inadvertent hysterectomy may be categorized into five groups based on the extent of residual disease present after surgery (Table 8). Tumors that invade less than 3 mm usually require no additional treatment. As the depth of invasion increases, the risk of having microscopic disease in paracervical tissues or pelvic lymph nodes increases. Most patients who have more than 4–5 mm of invasion should have additional local-regional treatment. Although some oncologists have advocated a radical excision of the parametrial tissues and pelvic lymph nodes, pelvic radiation therapy is used more commonly in this situation. Radiation therapy can prevent recurrence in most patients with group I and II disease.106 Treatment of patients with positive margins and particularly those with gross residual disease is compromised by the loss of the uterus and the inability to deliver optimal ICRT, although a high percentage of patients can still be cured with radiation therapy. Today, concurrent chemoradiation should be considered in the treatment of patients with group III to V disease, extrapolating from the results of randomized trials in other patients with high-risk cervical cancer.
Table 8. Prognostic grouping of patients after inadvertent simple hysterectomy for invasive carcinoma of the cervix
I | Microinvasive |
II | Disease confined to cervix; surgical margins negative |
III | Surgical margins positive; no gross residual disease |
IV | Gross residual disease |
V | Referred for treatment more than 6 months after diagnosis |
TREATMENT OF RECURRENT AND METASTATIC DISEASE
Localized recurrences of surgically treated cervical cancer are frequently curable if the disease was not previously treated with radiation therapy. In this situation, aggressive treatment is usually required with EBRT and either brachytherapy or carefully planned conformal radiation therapy.107, 108 Concurrent chemoradiation may be indicated to maximize the likelihood of local control.108 The prognosis is best for patients whose recurrent disease is confined to the central pelvis, with survival rates as high as 60–70%. Five-year survival rates are much lower (usually 15–20%) for patients with recurrences that involve the pelvic wall. However, some patients can be cured with radiation therapy alone, and it is possible that the use of chemoradiation with conformal radiation techniques that permit safer delivery of high-dose radiation to lateral pelvic structures will improve these results.
Radiation therapy is also a useful tool in the palliative treatment of patients with incurable metastatic cervical cancer. Local radiation therapy is particularly effective in the management of bleeding, bone pain, and compression of vital structures by enlarging soft tissue masses. Treatment is most likely to be effective when tumor is found outside previously irradiated areas, although re-treatment of a limited volume is occasionally possible. Symptoms can usually be relieved with a short course of treatment designed to maximize relief while minimizing side effects and limiting inconvenience to the patient.
REFERENCES
Barillot I, Horiot JC, Pigneux J, et al: Carcinoma of the intact uterine cervix treated with radiotherapy alone: A French cooperative study: Update and multivariate analysis of prognostics factors. Int J Radiat Oncol Biol Phys 38:969, 1997 |
|
Coia L, Won M, Lanciano R, et al: The Patterns of Care Outcome Study for cancer of the uterine cervix. Results of the second national practice survey Cancer 66:2451, 1990 |
|
Lowrey GC, Mendenhall WM, Million RR: Stage IB or IIA-B carcinoma of the intact uterine cervix treated with irradiation: a multivariate analysis. Int J Radiat Oncol Biol Phys 24:205, 1992 |
|
Perez CA, Grigsby PW, Nene SM, et al: Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer 69:2796, 1992 |
|
Keys HM, Bundy BN, Stehman FB, et al: Cisplatin, radiation, and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340:1154, 1999 |
|
Morris M, Eifel PJ, Lu J, et al: Pelvic radiation with concurrent chemotherapy compared with pelvic and paraaortic radiation for high-risk cervical cancer. N Engl J Med 340:1137, 1999 |
|
Rose PG, Bundy BN, Watkins J, et al: Concurrent cisplatin-based chemotherapy and radiotherapy for locally advanced cervical cancer. N Engl J Med 340:1144, 1999 |
|
Whitney CW, Sause W, Bundy BN, et al: A randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 17:1339, 1999 |
|
Peters WA III, Liu PY, Barrett RJ, II, et al: Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 18:1606, 2000 |
|
Eifel PJ, Jhingran A, Bodurka D, et al: Correlation of smoking history and other patient characteristics with major complications of radiation for cervical cancer. J Clin Oncol 20:3651, 2002 |
|
Grigsby PW, Perez CA, Kuske RR, et al: Adenocarcinoma of the uterine cervix: lack of evidence for a poor prognosis. Radiother Oncol 12:289, 1988 |
|
Eifel PJ, Burke TW, Morris M, et al: Adenocarcinoma as an independent risk factor for disease recurrence in patients with Stage IB cervical carcinoma. Gynecol Oncol 59:38, 1995 |
|
Berek JS, Hacker NS, Fu YS, et al: Adenocarcinoma of the uterine cervix: histologic variables associated with lymph node metastasis and survival. Obstet Gynecol 65:46, 1985 |
|
Korhonen MO: Adenocarcinoma of the uterine cervix: Prognosis and prognostic significance of histology. Cancer 53:1760, 1984 |
|
Morris M, Gershenson DM, Eifel PJ, et al: Treatment of small cell carcinoma of the cervix with cisplatin, doxorubicin, and etoposide. Gynecol Oncol 47:62, 1992 |
|
Abeler VM, Holm R, Nesland JM, et al: Small cell carcinoma of the cervix. A clinicopathologic study of 26 patients Cancer 73:672, 1994 |
|
Albores-Saavedra J, Gersell D, Gilks CB, et al: Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Arch Pathol Lab Med 121:34, 1997 |
|
Benedet J, Odicino F, Maisonneuve P, et al: Carcinoma of the cervix uteri. J Epidemiol Biostat 3:5, 1998 |
|
Heller PB, Malfetano JH, Bundy BN, et al: Clinical-pathologic study of stage IIB, III, and IVA carcinoma of the cervix: Extended diagnostic evaluation for paraaortic node metastasis—A Gynecologic Oncology Group study. Gynecol Oncol 38:425, 1990 |
|
Yang WT, Lam WW, Yu MY, et al: Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol 175:759, 2000 |
|
Grigsby PW, Siegel BA, Dehdashti F: Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol 19:3745, 2001 |
|
Grigsby PW, Siegel BA, Dehdashti F, et al. Posttherapy [18F] fluorodeoxyglucose positron emission tomography in carcinoma of the cervix: response and outcome. J Clin Oncol 22:2167;2004. |
|
Wharton JT, Jones HWI, Day T, et al: Preirradiation celiotomy and extended field irradiation for invasive carcinoma of the cervix. Obstet Gynecol 49:333, 1977 |
|
Piver MS, Barlow JJ, Krishnamsetty R: Five-year survival (with no evidence of disease) in patients with biopsy-confirmed aortic node metastases from cervical carcinoma. Am J Obstet Gynecol 139:575, 1981 |
|
Weiser EB, Bundy BN, Hoskins WJ, et al: Extraperitoneal versus transperitoneal selective paraaortic lymphadenectomy in the pretreatment surgical staging of advanced cervical carcinoma (a Gynecologic Oncology Group study). Gynecol Oncol 33:283, 1989 |
|
Possover M, Krause N, Plaul K, et al: Laparoscopic para-aortic and pelvic lymphadenectomy: Experience with 150 patients and review of the literature. Gynecol Oncol 71:19, 1998 |
|
Schlaerth J, Spiritos N, Carson LF, et al: Laparoscopic retroperitoneal lymphadenectomy followed by immediate laparotomy in women with cervical cancer: A Gynecologic Oncology Group study. Gynecol Oncol 85:81, 2002 |
|
American Joint Committee on Cancer: Cervix uteri. In Greene FL, Page DL, Fleming ID, et al (eds): Manual for Staging of Cancer. pp. 151, 156 6th ed.. New York, Springer-Verlag, 2002 |
|
Piver M, Barlow J, Vongtama V, et al: Hydroxyurea as a radiation sensitizer in women with carcinoma of the uterine cervix. Am J Obstet Gynecol 129:379, 1977 |
|
Perez C, Kurman R, Stehman F, et al: Uterine cervix. In Hoskins W, Perez C, Young R (eds): Principles and Practice of Gynecologic Oncology. pp 591, 662 Philadelphia, Lippincott, 1992 |
|
Logsdon MD, Eifel PJ: FIGO stage IIIB squamous cell carcinoma of the uterine cervix: An analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys 43:763, 1999 |
|
Grigsby PW, Perez CA: Radiotherapy alone for medically inoperable carcinoma of the cervix: Stage IA and carcinoma in situ. Int J Radiat Oncol Biol Phys 21:375, 1991 |
|
Berman ML, Keys H, Creasman W, et al: Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes (a Gynecologic Oncology Group study). Gynecol Oncol 19:8, 1984 |
|
Podczaski E, Stryker JA, Kaminski P, et al: Extended-field radiation therapy for carcinoma of the cervix. Cancer 66:251, 1990 |
|
Ballon SC, Berman ML, Lagasse LD, et al: Survival after extraperitoneal pelvic and paraaortic lymphadenectomy and radiation therapy in cervical carcinoma. Obstet Gynecol 57:90, 1981 |
|
Kim PY, Monk BJ, Chabra S, et al: Cervical cancer with paraaortic metastases: Significance of residual paraaortic disease after surgical staging. Gynecol Oncol 69:243, 1998 |
|
Brookland RK, Rubin S, Danoff BF: Extended field irradiation in the treatment of patients with cervical carcinoma involving biopsy proven para-aortic nodes. Int J Radiat Oncol Biol Phys 10:1875, 1984 |
|
Komaki R, Mattingly RF, Hoffman RG, et al: Irradiation of para-aortic lymph node metastases from carcinoma of the cervix or endometrium: Preliminary results. Radiology 147:245, 1983 |
|
Cunningham M, Dunton C, Corn B, et al: Extended-field radiation therapy in early-stage cervical carcinoma: Survival and complications. Gynecol Oncol 43:51, 1991 |
|
Rubin SC, Brookland R, Mikuta JJ, et al: Para-aortic nodal metastases in early cervical carcinoma: Long-term survival following extended-field radiotherapy. Gynecol Oncol 18:213, 1984 |
|
Haie C, Pejovic MH, Gerbaulet A, et al: Is prophylactic para-aortic irradiation worthwhile in the treatment of advanced cervical carcinoma? Results of a controlled clinical trial of the EORTC radiotherapy group Radiother Oncol 11:101, 1988 |
|
Rotman M, Choi K, Guze C, et al: Prophylactic irradiation of the para-aortic lymph node chain in Stage 2B and bulky Stage IB carcinoma of the cervix, initial treatment results of RTOG 7920. Int J Radiat Oncol Biol Phys 19:513, 1990 |
|
Delclos L, Fletcher GH, Moore EB, et al: Minicolpostats, dome cylinders, other additions and improvements of the Fletcher-Suit afterloadable system: Indications and limitations of their use. Int J Radiat Oncol Biol Phys 6:1195, 1980 |
|
Delclos L, Fletcher GH, Sampiere V, et al: Can the Fletcher gamma ray colpostat system be extrapolated to other systems? Cancer 41:970, 1978 |
|
Fyles AW, Pintilie M, Kirkbride P, et al: Prognostic factors in patients with cervix cancer treated by radiation therapy: Results of a multiple regression analysis. Radiother Oncol 35:107, 1995 |
|
Lanciano RM, Pajak TF, Martz K, et al: The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: A Patterns-of-Care Study. Int J Radiat Oncol Biol Phys 25:391, 1993 |
|
Perez CA, Grigsby PW, Castro-Vita H, et al: Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy Int J Radiat Oncol Biol Phys 32:1275, 1995 |
|
International Commission on Radiation Units andMeasurements: Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology. Bethesda, MD, International Commission on Radiation Units andMeasurements, 1985 |
|
Fletcher GH: Female pelvis. In Fletcher GH (ed): Textbook of Radiotherapy. Philadelphia, PA, Lea & Febiger, 1980 |
|
Chassagne C, Horiot JC: Propositions pour une définition commune des points de référence en curiethérapie gynécologique. J Radiol Electrol 58:371, 1977 |
|
Ling CC, Schell MC, Working KR, et al: CT-assisted assessment of bladder and rectum dose in gynecological implants. Int J Radiat Oncol Biol Phys 13:1577, 1987 |
|
Schoeppel SL, Fraass BA, Hopkins MP, et al: A CT-compatible version of the Fletcher system intracavitary applicator: Clinical application and 3-dimensional treatment planning. Int J Radiat Oncol Biol Phys 17:1103, 1989 |
|
Hall E, Brenner D: The dose-rate effect revisited: Radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys 21:1403, 1991 |
|
Eifel PJ: High dose-rate brachytherapy for carcinoma of the cervix: High tech or high risk? Int J Radiat Oncol Biol Phys 24:383, 1992 |
|
Eifel PJ, Moughan J, Owen JB, et al: Patterns of radiotherapy practice for patients with squamous carcinoma of the uterine cervix. A Patterns of Care study Int J Radiat Oncol Biol Phys 43:351, 1999 |
|
Nag S, Orton C, Young D, et al: The American brachytherapy society survey of brachytherapy practice for carcinoma of the cervix in the United States. Gynecol Oncol 73:111, 1999 |
|
Petereit DG, Sarkaria JN, Potter DM, et al: High-dose-rate versus low-dose-rate brachytherapy in the treatment of cervical cancer: Analysis of tumor recurrence—The University of Wisconsin experience. Int J Radiat Oncol Biol Phys 45:1267, 1999 |
|
Erickson B, Gillin MT: Interstitial implantation of gynecologic malignancies. J Surg Oncol 66:285, 1997 |
|
Eisbruch A, Johnston CM, Martel MK, et al: Customized gynecologic interstitial implants: CT-based planning, dose evaluation, and optimization aided by laparotomy. Int J Radiat Oncol Biol Phys 40:1087, 1998 |
|
Hughes-Davies L, Silver B, Kapp D: Parametrial interstitial brachytherapy for advanced or recurrent pelvic malignancy: The Harvard/Stanford experience. Gynecol Oncol 58:24, 1995 |
|
Monk BJ, Tewari K, Burger RA, et al: A comparison of intracavitary versus interstitial irradiation in the treatment of cervical cancer. Gynecol Oncol 67:241, 1997 |
|
Alberts DS, Garcia D, Mason-Liddil N: Cisplatin in advanced cancer of the cervix: An update. Semin Oncol 18(Suppl 3):11, 1991 |
|
Chauvergne J, Rohart J, Héron JF, et al: Essai randomisé de chimiothérapie initiale dans 151 carcinomes du col utérin localement étendus (T2b-N1, T3b, MO). Bull Cancer 77:1007, 1990 |
|
Kumar L, Kaushal R, Nandy M, et al: Chemotherapy followed by radiotherapy versus radiotherapy alone in locally advanced cervical cancer: A randomized study. Gynecol Oncol 54:307, 1994 |
|
Leborgne F, Leborgne JH, Doldán R, et al: Induction chemotherapy and radiotherapy of advanced cancer of the cervix: A pilot study and phase III randomized trial. Int J Radiat Oncol Biol Phys 37:343, 1997 |
|
Souhami L, Gil R, Allan S, et al: A randomized trial of chemotherapy followed by pelvic radiation therapy in stage IIIB carcinoma of the cervix. Int J Radiat Oncol Biol Phys 9:970, 1991 |
|
Tattersall MHN, Ramirez C, Coppleson M: A randomized trial comparing platinum-based chemotherapy followed by radiotherapy vs. radiotherapy alone in patients with locally advanced cervical cancer Int J Gynecol Cancer 2:244, 1992 |
|
Tattersall MHN, Larvidhaya V, Vootiprux V, et al: Randomized trial of epirubicin and cisplatin chemotherapy followed by pelvic radiation in locally advanced cervical cancer. Am J Clin Oncol 13:444, 1995 |
|
Tannock IF, Browman G: Lack of evidence for a role of chemotherapy in the routine management of locally advanced head and neck cancer. J Clin Oncol 4:1121, 1986 |
|
Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of Radiation Therapy Oncology Group trial (RTOG) 90-01. J Clin Oncol 22:872; 2004 |
|
Pearcey R, Brundage M, Drouin P, et al: Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol 20:966, 2002 |
|
Stehman FB, Ali S, Keys HM, et al. Radiation therapy with or without weekly cisplatin for bulky stage IB cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol 197:503.e1; 2007. |
|
Hreshchyshyn MM, Aron BS, Boronow RC, et al: Hydroxyurea or placebo combined with radiation to treat stages IIIB and IV cervical cancer confined to the pelvis. Int J Radiat Oncol Biol Phys 5:317, 1979 |
|
Sundfør K, Tropé CG, Högberg T, et al: Radiotherapy and neoadjuvant chemotherapy for cervical carcinoma. A randomized multicenter study of sequential cisplatin and 5-fluorouracil and radiotherapy in advanced cervical carcinoma stage 3B and 4A Cancer 77:2371, 1996 |
|
Wong LC, Ngan HY, Cheung AN, et al: Chemoradiation and adjuvant chemotherapy in cervical cancer. J Clin Oncol 17:2055, 1999 |
|
Thomas G, Dembo A, Ackerman I, et al: A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol 69:137, 1998 |
|
Lanciano R, Calkins A, Bundy BN, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluoruracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J Clin Oncol 23:8289 (2005) |
|
Monk BJ, Wang J, Im S, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/RadiationTherapy Oncology Group trial. Gynec Oncol 96:721; 2005. |
|
Ramirez PT, Levenback C, Burke TW, et al: Sigmoid perforation following radiation therapy in patients with cervical cancer. Gynecol Oncol 82:150, 2001 |
|
Levenback C, Eifel PJ, Burke TW, et al: Hemorrhagic cystitis following radiotherapy for stage Ib cancer of the cervix. Gynecol Oncol 55:206, 1994 |
|
Durrance FY, Fletcher GH, Rutledge FN: Analysis of central recurrent disease in stages I and II squamous cell carcinomas of the cervix on intact uterus. Am J Roentgenol 106:831, 1969 |
|
Eifel PJ, Morris M, Wharton JT, et al: The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 29:9, 1994 |
|
Horiot JC, Pigneux J, Pourquier H, et al: Radiotherapy alone in carcinoma of the intact uterine cervix according to G. H. Fletcher guidelines: A French cooperative study of 1383 cases Int J Radiat Oncol Biol Phys 14:605, 1988 |
|
Thoms WW, Eifel PJ, Smith TL, et al: Bulky endocervical carcinomas: A 23-year experience. Int J Radiat Oncol Biol Phys 23:491, 1992 |
|
Mendenhall WM, McCarty PJ, Morgan LS, et al: Stage IB-IIA-B carcinoma of the intact uterine cervix greater than or equal to 6 cm in diameter: Is adjuvant extrafascial hysterectomy beneficial? Int J Radiat Oncol Biol Phys 21:899, 1991 |
|
Perez CA, Kao MS: Radiation therapy alone or combined with surgery in the treatment of barrel-shaped carcinoma of the uterine cervix (stages IB, IIA, IIB). Int J Radiat Oncol Biol Phys 11:1903, 1985 |
|
Keys H, Bundy B, Stehman F, et al: Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol 89:341, 2003. |
|
Bush R: The significance of anemia in clinical radiation therapy. Int J Radiat Oncol Biol Phys 12:2047, 1986 |
|
Grogan M, Thomas GM, Melamed I, et al: The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 86:1528, 1999 |
|
Girinski T, Pejovic-Lenfant M, Bourhis J, et al: Prognostic value of hemoglobin concentrations and blood transfusions in advanced carcinoma of the cervix treated by radiation therapy: results of a retrospective study of 386 patients. Int J Radiat Oncol Biol Phys 16:37, 1989 |
|
Thomas G: The effect of hemoglobin level on radiotherapy outcomes: The Canadian experience. Semin Oncol 28:60, 2001 |
|
Höckel M, Vorndran B, Schlenger K, et al: Tumor oxygenation: A new predictive parameter in locally advanced cancer of the uterine cervix. Gynecol Oncol 51:141, 1993 |
|
Thomas GM, Dembo AJ: Is there a role for adjuvant pelvic radiotherapy after radical hysterectomy in early stage cervical cancer? Int J Gynecol Cancer 1:1, 1991 |
|
Morrow CP: Is pelvic radiation beneficial in the postoperative management of Stage Ib squamous cell carcinoma of the cervix with pelvic node metastases treated by radical hysterectomy and pelvic lymphadenectomy? Gynecol Oncol 10:105, 1980 |
|
Boyce J, Fruchter R, Nicastri A, et al: Prognostic factors in stage I carcinoma of the cervix. Gynecol Oncol 12:154, 1981 |
|
Burke TW, Hoskins WJ, Heller PB, et al: Prognostic factors associated with radical hysterectomy failure. Gynecol Oncol 26:153, 1987 |
|
Delgado G, Bundy B, Zaino R, et al: Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol Oncol 38:352, 1990 |
|
Nahhas WA, Sharkey FE, Whitney CW, et al: The prognostic significance of vascular channel involvement and deep stromal penetration in early cervical carcinoma. Am J Clin Oncol 6:239, 1983 |
|
Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology study. Int J Radiat Oncol Biol Phys 65:169, 2006. |
|
Kjorstad KE, Martimbeau PW, Iversen T: Stage IB carcinoma of the cervix, the Norwegian Radium Hospital: Results and complications. III. Urinary and gastrointestinal complications Gynecol Oncol 15:42, 1983 |
|
Landoni F, Maneo A, Colombo A, et al: Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 350:535, 1997 |
|
Sheu M, Chang C, Wang J, et al: MR staging of clinical stage I and IIa cervical carcinoma: A reappraisal of efficacy and pitfalls. Eur J Radiol 38:225, 2001 |
|
Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 52:1330; 2002. |
|
Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gyneclology patients treated with intensity-moldulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys 56:1354; 2003. |
|
Roman L, Morris M, Eifel P, et al: Reasons for inappropriate simple hysterectomy in the presence of invasive cancer of the cervix. Obstet Gynecol 79:485, 1992 |
|
Roman LD, Morris M, Mitchell MF, et al: Prognostic factors for patients undergoing simple hysterectomy in the presence of invasive cancer of the cervix. Gynecol Oncol 50:179, 1993 |
|
Ijaz T, Eifel PJ, Burke T, et al: Radiation therapy of pelvic recurrence after radical hysterectomy for cervical carcinoma. Gynecol Oncol 70:241, 1998 |
|
Thomas GM, Dembo AJ, Black B, et al: Concurrent radiation and chemotherapy for carcinoma of the cervix recurrent after radical surgery. Gynecol Oncol 27:254, 1987 |